

Theoretical calculations combined with various contrast experiments (temperature and pH) demonstrate that the selectively electrocatalytic capacity of chiral CDs toward Trp isomers is due to the different hydrogen-bond interactions between chiral CDs and Trp. L type of CDs (LCDs) is more likely to catalyze L-tryptophan (Trp) than D-Trp with the selective factor (/ L// D) of 1.60, whereas the D type of CDs (DCDs) tends to catalyze D-Trp (/ L// D: 0.63). In the pyranose form of glucose, carbon-1 is chiral. These chiral CDs have been demonstrated that they have selective capacity for electrocatalytic oxidization of tryptophan enantiomers. When there is more than one chiral center in a carbohydrate, look at the chiral carbon farthest from. Owens Corning FOAMULAR & FOAMULAR NGX 250 Extruded Polystyrene (XPS) Rigid Foam Insulation is a closed cell, highly. The formation of chiral CDs involves the processes of isomerization and aldol condensation. Herein, we report a facile one step base-catalyzed aldol condensation to fabricate the chiral CDs from glucose at ambient temperature and pressure. D-glucose has 4 chiral carbon atoms (24 16 possible stereoisomers) the name D-glucose implies just one of those stereoisomers one stereoisomer is the enantiomer of D-glucose the other 14 stereoisomers are diastereomers of D-glucose the reference for D & L designation of stereochemistry write all chiral centers in Fischer. Chiral carbon dots (CDs) as carbon-based chiral catalysts show great potential in chiral catalysis. I know that more than 99.5 of glucose exists as Haworth Projection - Closed Ring Structure and hence Linear - Fischer Projection practically does. However, I do not undertstand why that is, since the alleged chiral atoms do not have four different substituents: For example, the 3rd carbon atom is bound to one hydrogen atom, one hydroxylgroup and two neighbouring carbon atoms. Fischer Projection: 4 chiral centres Haworth Projection: 5 chiral centres My doubt is that is there any (specific) method (practical) to ascertain the number of chiral centres in both the forms. This explains why we can get energy from the starch in potatoes and other plants but not from cellulose, even though both starch and cellulose are polysaccharides composed of glucose molecules linked together.Chiral catalysis is one of the most direct and effective approach to obtain pure optical enantiomers. Glucose: Glucose has 4 chiral carbon both in ring and open structure marked with star (). I am aware that the open-chain form of glucose has four chiral carbon atoms. glucose, mannose, galactose, allose, altrose, idose, glucose, and talose each. The difference between the α and the β forms of sugars may seem trivial, but such structural differences are often crucial in biochemical reactions. carbon atoms (or chiral carbon atom) and are optically active. Any group written to the right in a Fischer projection appears below the plane of the ring in a Haworth projection, and any group written to the left in a Fischer projection appears above the plane in a Haworth projection.
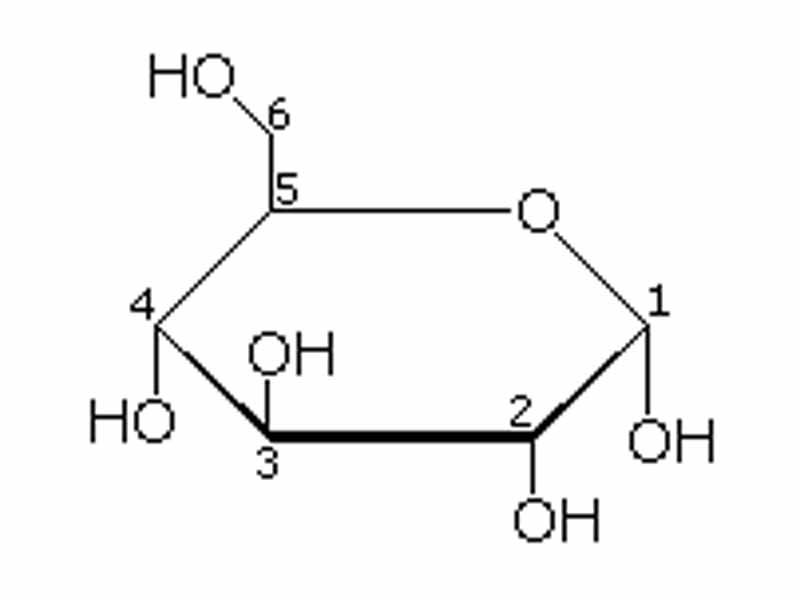
D-sugars predominate in nature, though L-forms of some sugars, such as fucose, do exist. All D-sugars have the OH on the right side and L-sugars have the OH on the left side. carbon, pyranose, sugar, heterocyclic, ring, hemiacetal, hemiketal, lactol, hydroxyl, carbonyl, aqueous, glucose, axial, equatorial, glucopyranose, chiral. 9 Glucose and fructose give positive Tollen's test as they reducing sugar because of the. The structure is simplified to show only the functional groups attached to the carbon atoms. D and L designations of sugars are based on the position of the hydroxyl on the chiral carbon farthest from the carbonyl group in the Fischer projection of the molecule. carbon is towards right and hence, all have D-configuration. The molecules are drawn as planar hexagons with a darkened edge representing the side facing toward the viewer. It is the source of energy in cell function, and the regulation of its metabolism is of great importance ( see fermentation gluconeogenesis ). All are known - some occur naturally and the others have been synthesized (see Table 20-1).
#Chiral carbon in glucose free
It is found in fruits and honey and is the major free sugar circulating in the blood of higher animals. Glucose is an aldohexose, which means that it is a six-carbon sugar with a terminal aldehyde group, shown by 1: The carbons labeled with an asterisk in 1 are chiral thus there are 2 4, or sixteen, possible configurational isomers. \)) the cyclic forms of sugars are depicted using a convention first suggested by Walter N. D and L notation is determined based on the chiral carbon farthest from the CO carbonyl group (penultimate carbon). Glucose (from Greek glykys sweet) has the molecular formula C 6 H 12 O 6.


 0 kommentar(er)
0 kommentar(er)
